Overview
STEM careers
Time
Materials
Per Group of 3-4 Students:
- Quart-sized zip top plastic bag
- Gallon-sized zip top plastic bag
- Ice cubes
- Salt
- 1⁄2 cup half & half
- 1⁄2 cup whipping cream
- 3 1⁄2 T sugar
- 1⁄4 t vanilla (optional)
- Rubber spatula
- Mixing spoons
- Plastic spoons
- Small cups
- Towels or paper towels
Instructions
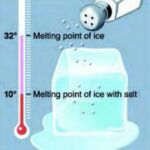
Students experience the importance of lowering the melting point of ice via the addition of salt, as well as the effect of continuously mixing ingredients to expose them to the coldest temperatures, as they make homemade ice cream.
Caution: Before starting this activity, check with the participants if they have allergies to any of the food ingredients used.
- Instruct children to measure the ingredients (younger students may need help). Put half & half, whipping cream, sugar, and vanilla in the smaller bag. Zip it and put it in the bigger bag.
- Pack ice in the big bag. Pour at least 1⁄4 cup salt evenly over ice. Seal the bag. (Explain that ice by itself isn’t cold enough to freeze ice cream, but adding the salt makes the ice a much lower temperature.)

- Each student in the group takes a turn shaking the bag, possibly with a towel around it because it gets very cold. Shake the bag for about 15 minutes altogether. (Shaking brings warmer ingredients in the middle into contact with the cold and speeds up the freezing. It also mixes the ingredients with air, making the ice cream soft and light.)
- If you don’t have ice cream yet, pour out melted ice cubes and insert more ice cubes and salt. Continue shaking.
- Remove the small bag of ice cream, rinse it off, and divide it into the small cups. Enjoy!
Guiding questions
-
Before they are put into the small bag, are the ingredients ice cream yet? Why not?
-
What is happening to the liquid in the small bag when you shake it? How can you tell?
-
How might adding chocolate or candy affect the ice cream making process?
-
If you let the ice cream melt, would it be exactly the same as when you first mixed the ingredients together?
Engineering & science connections
- Engineers invented refrigeration using the principles of heat transfer and phase changes (liquid to gas and gas to liquid). Before people had refrigerators, starting about 100 years ago, they had to store food in ice boxes. They also had to make ice cream by hand, like the students just did.
- When a liquid changes to a gas it absorbs heat. This process done over and over can remove heat from a system. An example of this is airconditioners using Freon gas to create cold temperatures.
- Using the principle of heat transfer, engineers are able to cool all kinds of things. For example, they invented machines to make ice in skating rinks and artificial snow for skiing.
The American Society of Heating, Refrigerating and Air-Conditioning Engineers, Chair of Engineers Week 2011, helps keep indoor environments comfortable, preserve the outdoor environment, and deliver healthy food to consumers.

0 Comments