Overview
Materials
Per Class:
|
Per Small Group:
|
Instructions
Students create an electrical circuit and test whether various liquids conduct electricity.
PREPARATION:
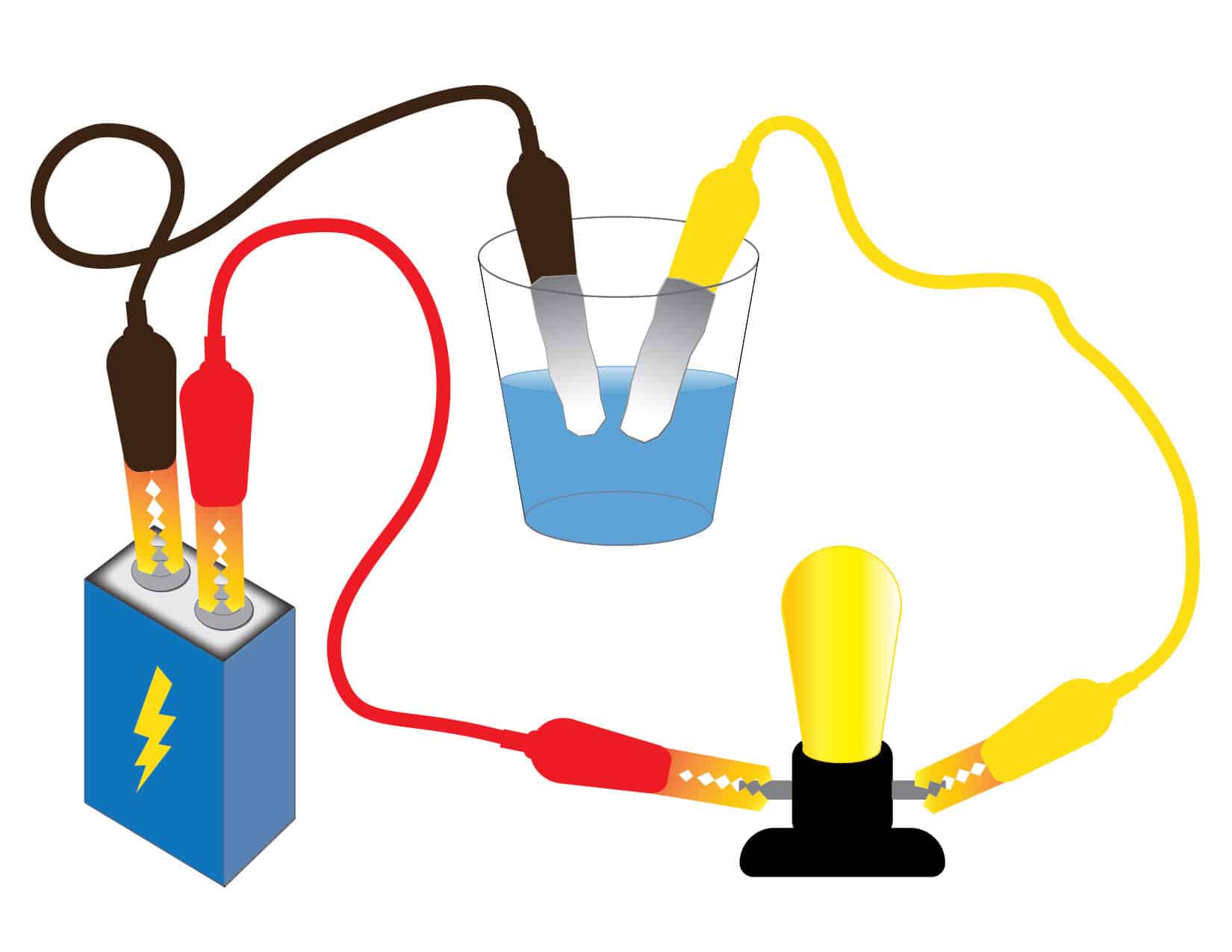
Recommendation: Prepare your own closed circuit and set of liquids and conduct your own tests ahead of time. To prepare the circuit:
- Connect a wire between the negative terminal of the battery and one side of the bulb holder.
- Connect another wire between the positive terminal of the battery and the other side of the bulb holder.
Decide whether to prepare the cups of liquid ahead of time or let students set up their own cups. Students test the following liquids, but other liquids can be used (e.g., soda and vinegar):
- 150 ml water
- 150 ml water and 1 Tbsp. salt
- 150 ml water and 1 Tbsp. sugar
- 150 ml Gatorade
- 150 ml chocolate milk
- 150 ml orange juice
Prepare testing materials. If not using alligator clips, cut 8″ to 10″ pieces of wire and then strip the ends. Keep extra bulbs and batteries on hand.
ACTIVITY:
- Ask students: Is it possible to light up a lightbulb with wire and a battery? Let students respond with their ideas.
- Arrange students in groups and then give each group a bulb, bulb holder, 9-volt battery, battery holder, and two wires. Challenge students to create a circuit that will make the bulb light. Work with groups to help as needed, asking questions to spark their thinking.
- When students have their circuits working, invite them to try adding some other objects to their circuit: a penny, paper clip, or other metal object. (They will need to use a third wire.) Make sure students grasp that these are conductors, and that they understand the difference between conductors and insulators.
- Ask: Can a cup of liquid be a conductor? Explain that students will test liquids to see if they can conduct electricity. In order to do so, students will need to modify their circuit to create a conductivity tester.
- Give each group two large paper clips and two small pieces of aluminum foil. Instruct students to wrap foil around each paper clip so that it is completely covered (except for an end to attach a wire). These are their testing “paddles” that can go in liquid so that the clips or wires don’t get wet. Emphasize key safety points, such as keeping the clips and wires dry (and never putting electrical appliances in water).
- Tell students to attach the paddles to the end of the wires or alligator clips. The paddles will serve as conductivity testers. Instruct students to confirm that their testers work by touching the paddles together—their bulb should light up.
- Students prepare their six testing liquids or use those set up in advance. Elicit from students their predictions on which liquids will conduct electricity.
- Give students these testing guidelines:
- Demonstrate how to wipe off the testers between liquids.
- Instruct students not to touch the tester paddles together once they are placed in each liquid. If they do, the current will just go from paddle to paddle, and they won’t get accurate results about the liquid they are testing.
- Students place the tips of their testers inside each cup and then observe what happens, taking notes so that they are ready to talk about the results afterward.
- When students are finished testing, discuss the results. Students should discover that the saltwater, chocolate milk, and Gatorade conducted electricity best. Allow students to see the product labels, as available, to consider what these solutions contain. Make sure they realize that the solutions with salt work best, and point out why that is so.
Guiding questions
-
What happens when you connect all the wires in your circuit, or “close the circuit”?
-
What happens when you disconnect a wire?
-
What happens when you move the paddles closer together and further apart?
-
What other liquids would you like to try this experiment with? What are your predictions?
Engineering & science connections
- Conductor: Something that electric current can pass through easily. Examples: metals such as copper and aluminum.
- Insulator: Something that does not allow electricity to flow through it. Examples: rubber, plastic, glass, wood.
- Solution: A mixture of two or more substances, where one substance is completely dissolved in the other.
- When you add salt to a liquid like water, the salt molecules dissolve in the liquid and break into smaller parts called ions. The ions carry electrical charge through the liquid. Acids and bases also break down to form ions when dissolved in water. In fact, all water, with the exception of pure water, will conduct electricity because it contains ions that conduct the current.
- The use of liquid conductors opens the door to flexible electronic materials. This means that new things like clothes or car seats could be electrified. Other existing electronic devices could become flexible themselves. For example, there could be a cell phone with a huge display that could be rolled up and tucked away when not in use.
- Engineers are researching how to use liquid conductors to help make lithium ion—or rechargeable—batteries safer. Currently, a highly flammable solvent is used as a medium through which ions pass. If this solvent can be replaced with a high-sodium or acidic liquid, the risks of injury would be greatly reduced.
Courtesy of teacherstryscience.com
Developed by Kathleen Crowe of Texas Regional Collaboratives

0 Comments