Overview
Materials
Per Class:
|
Per Small Group:
|
Instructions
Students learn the properties of electricity and how a battery works through demonstrations and the building of a rudimentary battery.
Caution: For safety, be sure to inform participants not to taste or eat any of the materials during this activity.
- As an option, ask students to generate static electricity via one or more of the following:
- In leather shoes (no rubber sneakers) or socks, kids rub their feet on a thick carpet and touch a metal object or a friend’s arm to generate a spark.
- Rub a balloon on their hair and stick it to a wall.
- Run a comb through their hair and hold it over the confetti, which will jump to the comb.
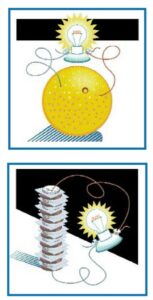
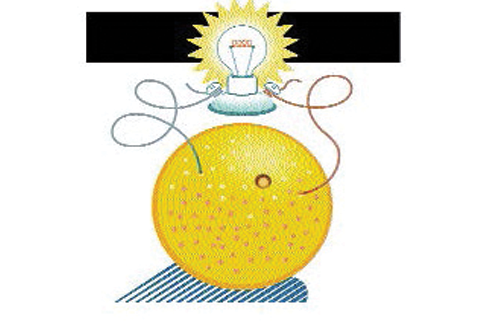
- To demonstrate an electrical current, as opposed to static electricity, connect a copper wire to one lead of a voltmeter or lightbulb, and a zinc wire to the other. Place the ends of each wire into the orange. Show the students that there is voltage in the voltmeter, or that the bulb is lighting up. Explain that electricity is caused by a chemical reaction when two dissimilar metals are placed in a salty solution.
- Ask kids what they think will happen if you try this with a banana. Conduct the demonstration with several different fruits, noting the different voltage or different brightness of the lightbulb that each creates.
- Ask kids to speculate on what this demonstration so far has to do with batteries. Tell them that they are going to make a very simple kind of battery now in the next part of this activity.
- Place students in small groups and hand out materials.
- Provide the following instructions:
- Place vinegar in a bowl. Soak each of the squares of paper towel in the vinegar.
- Make a pile of coins by alternating 10 pennies with 10 dimes, and a square of paper towel between each. (So: penny, paper towel square, dime, paper towel square, penny, etc.)
- Place one of the wires connected to the voltmeter or lightbulb at the top of the pile and another at the bottom. The lightbulb or voltmeter should register the charge.
- If students moisten one fingertip on each hand and hold the pile of coins between those two fingers, they should be able to feel a slight electric current
Guiding questions
-
Will your hair create static electricity if it’s wet and you run a comb through it?
-
How long do you think the balloon will stick to the wall?
-
What solution besides vinegar might work to make a simple battery?
-
Do you think you could power a toy with this battery? What would you have to do to make it work?
Engineering & science connections
- Electricity is a type of energy associated with the flow of electrons. Electrons are the negatively charged particles in atoms. Atoms are the smallest building blocks of matter. Under certain conditions, electrons can jump between atoms.
- Static electricity is caused when electrons build up in one place, which happens when you do things like rub a balloon in your hair. This buildup of electrons creates an electric charge, which jumps to a place with less electrons.
- When two different metals are placed in a salty solution, one tends to give up electrons in the solution, and one tends to collect them. This chemical reaction causes an electrical current.
- Alessandro Volta discovered how to create an electrical current using a piece of cardboard soaked in a salty liquid between a plate of zinc and a plate of copper. He called it a “Voltaic Cell.”
- Engineers at the company Tesla are developing new batteries to power your home that can be recharged during the day using solar cells. Batteries have been around for a long time, but still are being improved to provide more power for longer times in smaller packages.
Thank you to IEEE’s History Center for this activity. Students can learn more about Galvani, Volta, their discoveries, and inventions—and the engineers and inventions that came after them—at the IEEE Virtual Museum: ieee.org/museum.

0 Comments