Overview
STEM careers
Time
Materials
Per Group:
|
Per Participant:
Per Team:
|
Relevant Terminology
Climate change: Changes in long-term temperature, precipitation, and other weather conditions that are primarily brought about by the burning of fossil fuels.
Coral bleaching: A phenomenon wherein high water temperatures cause corals to expel the symbiotic algae in their bodies that keep them healthy. This is already happening in areas such as the Great Barrier Reef in Australia.
Ocean acidification: The process of ocean water becoming more acidic as carbon dioxide is dissolved in seawater to produce carbonic acid.
Introduce
GETTING READY
Assemble the supply bags for each team.
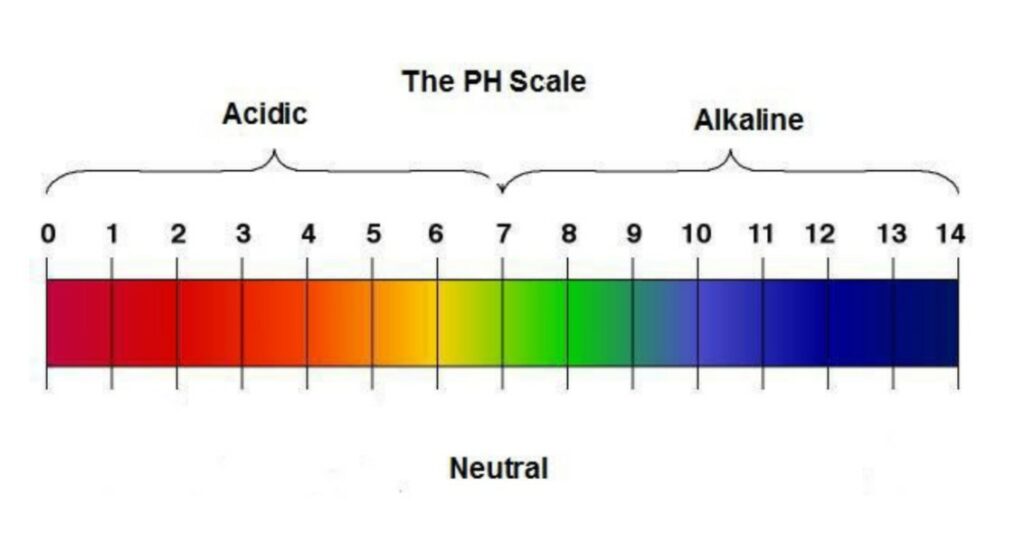
To set the context for this activity, be prepared to review the following topics with participants: acids, bases, the pH scale, acidification, and the role increasing levels of carbon dioxide play in climate change.
Purchase dry ice as close to the time of the activity as possible to minimize loss from sublimation. Store dry ice in an insulated cooler. If transporting dry ice by car, place the cooler in the trunk and open a window to keep fresh air flowing through the vehicle. Always wear protective gloves when handling dry ice. If pellets are not available, you can break a block of dry ice into pieces about the size of dice.
Familiarize yourself with the safe use of universal pH indicator. The diluted solution is not particularly dangerous, but it is an eye and skin irritant. From the American Chemical Society:
Be sure you and the students wear properly fitting goggles during the activity and wash hands afterwards. Universal pH indicator is alcohol-based and flammable. Read and follow all safety warnings on the label. At the end of the lesson, have students pour their used solutions in a waste container. Dispose of this waste down the drain or according to local regulations.
Tap water is acceptable for this activity, but test the water you plan to use in advance. Place a few drops of universal pH indicator in a beaker of water. Green means the water is neutral, while yellow or orange mean the water is acidic. If your tap water is acidic, use distilled water instead.
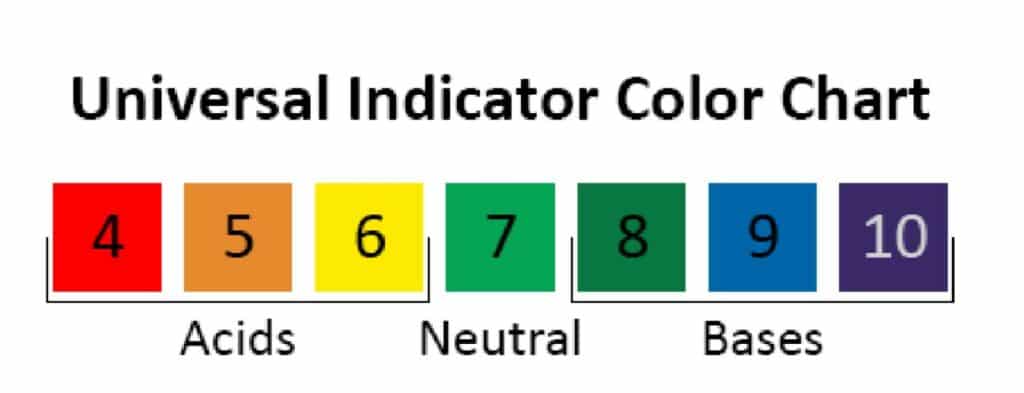
Acids turn universal indicator shades of yellow, orange, and red; bases turn dark green, blue, and violet. Credit: Carnegie Science Center.
INTRODUCTION
Ask questions to learn what participants already know about ocean acidification and climate change. Fill in gaps in their knowledge as needed.
- What are some things you know about acids and bases?
- What is a pH scale?
- Where do acids and bases fall on this scale?
- What is a common household acid? A common household base?
- How do you think climate change and atmospheric composition affect the oceans? (Warmer water and acidification affect all life in the ocean. Higher concentrations of carbon dioxide in the air cause air-water equilibrium to happen and increase the carbon dioxide in the ocean. Other carbon-containing pollutants such as methane and carbon monoxide also affect the ocean.)
As is appropriate for your participants, provide the following information, which includes how to learn more:
Rising carbon dioxide (CO2) concentrations in the atmosphere, combined with CO2 released from sea life respiration, can cause acidic conditions in the ocean. This can be harmful to corals, shellfish, and other marine creatures that are made of calcium carbonate, which is easily damaged (broken down) in an acidic environment. A Stanford University student named David A. Koweek developed a theory that using aeration to remove CO2 from coastal areas (1–3 meters deep) may combat this problem. This action would be similar to aerating a freshwater lake to prevent a type of water pollution called eutrophication. Koweek performed a bench scale test of his theory and was able to reduce the CO2 concentration in the seawater tank he used by 22%. Although this test was quite successful, aeration requires a large amount of energy, so its use is questionable for a full-scale application. Utilization of solar power or some other renewable energy may make it more applicable.
For more information, read the article “Bubbles could be a targeted remedy for ocean acidification” from Chemical & Engineering News, a publication of the American Chemical Society. cen.acs.org/articles/94/ web/2016/03/Bubbles-targeted-remedy-ocean-acidification.html
Instructions
Explain the challenge to participants: Design and build a system that uses bubbles to remove dissolved carbon dioxide from water. Before they get started, they will see a demonstration of how universal pH indicator works.
Demonstrate the behavior of the universal pH indicator:
- Fill the 1,000 mL beaker with 900–1,000 mL of water.
- Add several drops of universal pH indicator solution and stir (the liquid in the beaker should be green). The color should be vibrant enough that all participants can see it. If it’s not, add a few more drops of universal pH indicator.
- Explain that universal indicator is a chemical solution that changes color depending on its pH. Neutral is green, acids are yellow-red, and bases are blue-violet.
- Add acid (lemon juice or vinegar) to the beaker in small amounts and watch as the color changes from green to yellow to orange.
- Ask participants to predict what will happen when a base (baking soda) is added to the acidic solution. Add small amounts of baking soda and stir while noting the color change. The solution should go back to green, and then proceed to blue—indicating a base.
Divide the participants into teams of three or four. Distribute a pair of goggles to each person and one beaker to each team. Direct participants to fill their beakers about three-quarters full of water.
Explain that another phenomenon that participants need to understand before undertaking their challenge is ocean acidification. Introduce ocean acidification:
- Ask participants what they think will happen when carbon dioxide is added to the water. If participants think the pH will change, have them explain why they think this. It’s okay if the participants have no idea what will happen.
- Use the tongs to add a few pieces of dry ice to the teams’ beakers. The dry ice will sink and form bubbles in the liquid and create a layer of fog above the beaker. Notice that the color will change from green to yellow/ orange, indicating the carbon dioxide made the water acidic.
- Explain that the water became acidic because carbon dioxide reacts with water to form carbonic acid (which is also found in all carbonated beverages). As the level of carbon dioxide in the atmosphere rises through the burning of fossil fuels, it makes the oceans more acidic. This has far-reaching implications for various forms of life, both in the ocean and on land. Bubbling air through water helps to release dissolved carbon dioxide back to the atmosphere—thus making the water less acidic.
Give each team a bag of supplies. Remind them of the design challenge and add these details and constraints: Using the materials provided, work in your teams to design and build a system that uses bubbles to remove dissolved carbon dioxide from water. The constraints are as follows:
- You may not blow directly into the system to create bubbles.
- The bubbling should not cause water to spill out of the beaker.
- The universal indicator should change from orange/yellow to green, indicating a neutral pH.
- You do not have to use all the supplies.
Give the teams 20–30 minutes to brainstorm and build prototypes. Teams can test their prototype in their beaker of acidic water. Depending on the design and size of the bubbles, the color change can happen quickly or it may take several minutes.
Tip: Fine bubbles remove carbon dioxide faster than large bubbles, because with many small bubbles, you have a larger surface area than you do with a few larger ones. A larger surface area gives more area for the gas exchange to occur.
Evaluate the success of each design by asking teams the following questions:
- Were you able to return the water to neutral pH (green)?
- How long did it take to remove the excess carbon dioxide from the water?
ACTIVITY VARIATIONS
If you don’t have beakers, you can use clear plastic bottles with the tops cut off.
Guiding questions
GUIDANCE FOR YOUNGER CHILDREN
QUESTIONS TO ASK AFTER THE ACTIVITY
- How did you decide on which materials to use out of your supply bag?
- If your water didn’t become neutral on the first try, what do you think the problem was?
- What made your bubbler successful? How could it be improved?
GUIDANCE FOR OLDER YOUTH AND ADULTS
QUESTIONS TO ASK AFTER THE ACTIVITY
- Which of the materials in your supply bag turned out to be the most useful in meeting this challenge?
- What could you do to make more, smaller bubbles, so that the water attains a neutral pH more quickly?
- What ideas does this activity give you for reducing ocean acidification?
Engineering & science connections
GUIDANCE FOR YOUNGER CHILDREN
Engineering Connections
There are many challenges in using this technique to fight ocean acidification on a large scale. This bubbling technique would only work well in shallow, coastal waters where there is more carbon dioxide in the water than in the air. It would take a lot of electricity to run the pumps to make all the bubbles; if that power were made by burning fossil fuels, it would contribute to the problem we are trying to solve. Using renewable power sources might help make this idea a reality.
This technique has been successful in the lab, but it hasn’t been tried in the ocean. Engineers will need to build prototype bubblers in the ocean to test this idea further. It might work—or it might fail. But even if bubbling is a failure, at least we will know another way NOT to fix the problem of ocean acidification. Failure is always one step closer to success!
Science Connections
Most of our automobiles and electrical power plants run on fossil fuels: oil, coal, or gas. These fuels produce carbon dioxide (among other things) when burned. Over the last 150 years, the level of carbon dioxide in the atmosphere has been increasing. This has led to an increase in Earth’s average temperature and to the oceans becoming more acidic.
Acidic water makes it hard for corals to grow, meaning that coral reefs have a harder time rebuilding when they are damaged. This negatively affects fish and the people that depend on them.
GUIDANCE FOR OLDER YOUTH AND ADULTS
Engineering Connections
Engineers are finding that there are many challenges in using this technique to fight ocean acidification on a large scale. This bubbling technique would only work well in shallow, coastal waters where there is more carbon dioxide in the water than in the air. It would take a lot of electricity to run the pumps to make all those bubbles; if that power came from burning fossil fuels, it would contribute to the problem we are trying to solve. Using renewable power sources might help make this idea a reality.
This technique has been successful in the lab, but it hasn’t been tried in the ocean. Engineers will need to build prototypes in the ocean to test this idea further—and work with the scientists who monitor these ecosystems. We may find that bubbling is a useful tool in fighting ocean acidification, or these efforts might fail. Failure is a risk that all engineers face, but learning from failure is a critical part of the engineering design process.
Science Connections
Most of our automobiles and electrical power plants run on fossil fuels: oil, coal, or gas. These fuels produce carbon dioxide (among other things) when burned. Over the last 150 years, the level of carbon dioxide in the atmosphere has been increasing. This has led to an increase in Earth’s average temperature and to the oceans becoming more acidic.
Corals produce a skeleton of calcium carbonate, which dissolves in acid. Ocean acidification makes it harder for corals to grow and harder for them to recover after hurricanes or bleaching events, both of which are predicted to become worse as our climate warms. Since a quarter of all fish in the ocean spend part of their life on coral reefs, this problem also impacts the people who rely on these fish for food or income.
The source for this activity was developed by the Fleet Science Center, in association with MacGillivray Freeman Films and Pacific Life. All rights reserved.
Supplemental content adapted for Dream Big Activities by Carnegie Science Center.


0 Comments